News Room
Here are the latest news about Celltrion Pharm.
OUR BUSINESS
Celltrion Pharm runs businesses that elevate the value of life.
-
01ChemicalChemical
With production facilities and technologies on par with global standards, we supply safe and high-quality chemical products to the market
VIEW MORE -
02BiosimilarBiosimilar
We provide patients with opportunities to use high-quality biopharmaceutical products at reasonable prices.
VIEW MORE -
03CMOCMO
From pharmaceutical development to finished product manufacture, we offer customized contract manufacturing services using our global-standard pharmaceutical production facilities.
VIEW MORE
Product
Celltrion Pharm offers better opportunities and environments for treatment.

Godex Cap. is a hepatoprotective agent mainly consisting of carnitine that helps in mitochondria’s structure and functions. It was developed with the mixture that helps Hepadif and DDB achieve their effects. In particular, Godex Cap. is used for hepatic diseases related to elevated transaminase values.
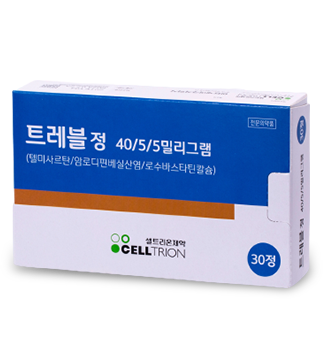
Treble Tab is a triple combination medicine that combines ARB telmisartan, CCB amlodipine, and RosuvastatinStatin, a hyperlipidemia medicine. It is used for hypertension patients with hyperlipidemia.

Remsima is a biosimilar of Infliximab, approved by the European Medicines Agency (EMA) in 2013 and the United States Foods and Drug Administration (FDA) in April 2016. It is used for the treatment of rheumarthritis, spondylitis ankylopoietica, ulcerative colitis ulcerativa, Crohn disease in adults and children, and psoriasis.
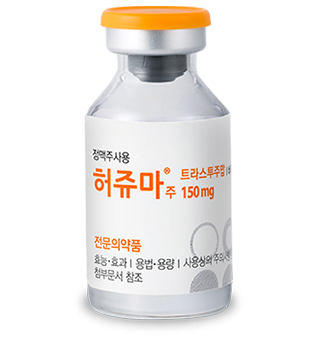
Herzuma® a biosimilar of Trastuzumab, approved by the European Medicines Agency (EMA) in February 2018 and the United States Foods and Drug Administration (FDA) in December 2018. It is used for the treatment of metastatic breast cancer with HER2 overexpression, early breast cancer, and metastatic gastric cancer.

Truxima® is a biosimilar of Rituximab, approved by the European Medicines Agency (EMA) in February 2017 and the United States Foods and Drug Administration (FDA) in 2018. It is used for the treatment of lymphoma, chronic lymphocytic leukemia, and rheumarthritis.